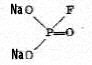
|
|
|||||||
Monofluorophosphate History |
|||||||
Fluorophosphates (among them sodium monofluorophosphate) were first prepared and described about 75 years ago. In the meantime they found a wide field of applications. A search for "fluorophosphate" or "monofluorophosphate" in one of the patent databases reveals more than 200 patents for either keyword. Some of them detail the manufacture of the compounds, while others provide compositions for usage as corrosion inhibitor, electrolyte in batteries, or as toe nail and finger nail fungus killer, to name a few examples. Most of the patents, however, are related to products for oral and dental care, which also reflects the fact that most people nowadays associate the terms "fluorophosphate" or "monofluorophosphate" with toothpaste. Accordingly, one would expect that information on the history of fluorophosphates (at least sodium monofluorophosphate, abbreviated as MFP or SMFP) should most probably be found in dental publications. Indeed, there were a few history articles written in the early 1980´s (1-3) on which more recent accounts still rely heavily: In a TV news program "Don´t swallow your toothpaste" (Health Alert Series in England´s Channel 4, June 19, 1997) it was claimed that John Hein was the person who developed MFP at the University of Rochester. John William Hein was a former student of Harold Carpenter Hodge, the Rochester scientist who worked -inter alia- for the Manhattan Engineer District (atomic bomb program). Mike McCoy, on the other hand, explains in a "Science and Technology" issue of April 16, 2001, that when Procter and Gamble was on top after invention of their famous product "Crest" (with stannous fluoride, 1955), "the next dental fluoride breakthrough --MFP-- was already in the works. Ozark had discovered the compound in 1949 and by the early 1960´s was selling it to toothpaste makers overseas. MFP really took off in 1967 when Colgate-Palmolive first launched Colgate with MFP, providing new competition for Crest and cementing fluoridated toothpaste as the marketplace standard." However, neither Ozark nor Hein did actually "discover" the compound. And when it was prepared for the first time it was not in the first place thought to be useful as a toothpaste additive. In fact, there have been fears for certain reasons that the substance was quite toxic, as Ozark´s Wayne E. White details in a review of the history of monofluorophosphate (1). And it is only here that one reads about the involvement of Dr. Willy Lange, who is often cited in another context -together with Gerda von Krueger- for the discovery of the highly toxic nature of certain organic fluorophosphates (so-called "nerve agents"). Wayne White writes : "First, there was the idea that had originated with Dr. Lange that a fluorophosphate should be effective as a mothproofing material and might therefore be toxic also to humans. But a more compelling basis for the belief that this might be quite poisonous was the knowledge that when the PO3F2- group is combined with two isopropyl groups to form the ester, diisopropyl-monofluorophosphate, known during and after World War II as DFP, it is a potent inhibitor of choline esterase and hence is one of the ´nerve gases´. The early studies by Shourie et al. (1950) immediately relieved us of the thought that we might have a very poisonous substance and that finding combined with the report on caries inhibition pointed the way toward dental and possibly medical applications" (1)
---
---
The following chronologic history which includes the relevant patents is not intended to make the stuff more poisonous than it actually is but to show how inventive some people can be.
1885/86 Reporting their experiments on a series of phosphorus fluorides, the French chemists Marcellin Berthelot and Henri Moissan suggested the possible formation of "fluorophosphite" or "fluophosphate" during hydrolysis of the gaseous compounds (7), yet neither of the two researchers followed up their ideas.
1898 R. F. Weinland and J. Alfa prepared and described some fluorosulfates and "fluorophosphates". While they were able to synthesize what they called potassium monofluorophosphate ("PO3FlHK . H2O"), they could not obtain a sodium or ammonium salt of the fluophosphoric acid, nor the salt of a metal of higher valence (8,9). Patents were filed soon which proposed the use of that substance for an electrolytic process for the recovery of tin from waste tinned metal and alloys (British Patent GB 24,203; Oct. 23, 1912) or for the protection of wool and textiles (British Patent GB 295,742; May 18, 1927). It was later realized, however, that "the so-called fluorophosphates of the older literature are not true fluorophosphates with anions containing the P-F bond, but phosphates, which as stated by Seifert, ..., should be written as KH2PO4 . HF" (9a).
1923 Willy Lange, a graduate student at the Chemistry Department of the Friedrich Wilhelms University of Berlin (nowadays called Humboldt University), prepared his Ph.D. thesis on the action of sulfuric acid on fluorspar. There is not only hydrogen fluoride developed, but fluosulfonic acid is formed which Lange examined at the instigation of his teacher Wilhelm Traube (10). By the time, Traube had already patented the manufacture of certain alkyl fluorosulfonates (11,12).
1927 Willy Lange described certain similarities in the chemical behavior of perchlorate, fluoborate and fluorosulfonate anions. In an attempt to prepare some new fluoro acids to compare their corresponding properties, he discovered the formation of fluorophosphates upon hydrolysis of phosphorus oxytrifluoride (POF3). The first in a series of hydrolysis products he observed was difluorophosphoric acid (13).
1928 Lange prepared difluorophosphoric acid by reacting phosphorus pentoxide (P2O5) with ammonium fluoride or by pouring P2O5 into hydrofluoric acid. As a side product of the reaction he found hexafluorophosphoric acid (14).
1929 By heating a solution of a difluorophosphate in a dilute sodium hydroxide solution, Lange found that one fluoride ion is lost from the complex and a monofluorophosphate (MFP) is formed, which in some aspects behaves similar to sulfate. Though he could prepare a number of monofluorophosphate salts (among them the sodium, ammonium, potassium, silver monofluorophosphates) he was unable to prepare the free monofluorophosphoric acid, which fact posed a challenge to him (15).
1930 Willy Lange patented the synthesis of aryl fluosulfonates from aryl diazonium fluosulfonates and proposed their use as insecticides: Willy LANGE, Berlin: "Verfahren zur Herstellung von Arylfluorsulfonaten und ihren Derivaten", German Patent DE 532.394, filed Aug. 8, 1930; issued Aug. 27, 1931 This patent clearly underlines Lange´s claim that "during my academic career in Berlin, my main research interest concerned the synthetic organic insecticides for agricultural purposes (at that time unknown)" (15a). The organic substances then in use as insecticides (Nicotine and Rotenone) were extracted from certain plants, had to be imported and were rather expensive.
1932 Gerda von Krueger, one of Lange´s students, became involved in fluorophosphate research as part of the work for her PhD thesis. She was able to prepare potassium and ammonium hexafluorophosphates in higher yields and forwarded some of these salts to Riedel de Haen Company for toxicological tests which showed them to be rather inert (16,17). In collaboration with Lange she also prepared a few esters of monofluorophosphoric acid and thus both felt sure the isolation of the free acid might be possible under conditions more favorable than the ones hitherto used. Upon heating silver monofluorophosphate with methyl or ethyl iodide they prepared the corresponding dialkyl monofluorophosphates which exerted a strong action on the authors: "the fumes of these compounds have a pleasant, slightly aromatic odor. But a few minutes after inhalation there´s a feeling of pressure to the larynx and difficulty in breathing. Then a disturbance of consciousness develops, as well as blurred vision and a painful oversensitivity of the eyes towards light. Only after several hours the problems wear off. They are apparently not caused by acidic products of a possible decomposition but by the esters themselves. The effects are exerted by very small amounts." Several homologues have been prepared, i.e. the di-n-propyl and the di-n-butyl esters (16). The amounts of the esters to which the human organism reacts in the way described are below one milligramm (17). Lange felt these compounds might be useful for pest control and offered them to I. G. Farben Industry for evaluation. But the company apparently had no interest at that time (17a). Within two years I. G. Farben changed its mind and began its own developmental program.
1934 At Germany´s IG Farbenindustrie, Leverkusen, chemist Gerhard Schrader was asked to develop new synthetic insecticides (18). Compounds of fluorine just had begun to attract the interest of chemists. New fluorine-containing dyes had been synthesized, the "freons" became famous, fluoroacetate was recommended for protection of wool, and inorganic fluorides had been patented as insecticides already. Moreover, through the Inorganic Chemistry Department of I. G. Farben hydrogen fluoride was available on an industrial scale. Therefore, several fluorine substituted organic acids as well as fluoroethanol and its derivatives were tested by Schrader and colleagues for insecticidal activity. While the compounds were of interest from a purely scientific viewpoint, none of them was considered to possibly be of practical use. Otto Bayer, then director of research at I. G. Farben, was disappointed and assigned Schrader to work in other fields (19).
1935 By 1935 the Friedrich-Wilhelms University of Berlin was under the strong influence of the Nazi-Regime and many students and teachers were either arrested or forced to leave. Lange, who since 1925 was married to Lilli Baermann (Sept. 6, 1901 - Feb. 1982), a colleague at the Chemistry Department (19a), was forced "for political reasons" (his wife was Jewish (19b)) to leave the academic career. With the approval of the Ministry of education, he first took a leave from his teaching job and moved to Duesseldorf, Germany, where he worked for Henkel & Cie. Henkel´s director at that time, chemist Dr. Hugo Henkel, a former graduate (1905) of the University of Berlin, was setting up a central chemical laboratory for the company to conduct research on new chemical products (20). Henkel was apparently not a friend of the Nazis, and it is claimed that none of his Jewish or half-Jewish workers became a victim of the holocaust. At I. G. Farben, Gerhard Schrader, Otto Bayer and Hans Kükenthal patented alkyl fluosulfonates as insecticides. The patent claims that alkyl fluosulfonates are more effective against insects than the aryl analogues proposed earlier (by Lange in 1930): Gerhard SCHRADER, Otto BAYER, Hans KÜKENTHAL, assignors to I. G. Farbenindustrie, Frankfurt: "Schädlingsbekämpfungsmittel", German Patent DE 664.062, filed April 4, 1935; issued August 19, 1938 On July 3, 1935, Schrader and Bayer filed a patent on Dialkylaminophosphofluorides to be used as insecticides: Gerhard SCHRADER, Otto BAYER, assignors to I. G. Farbenindustrie, Frankfurt: "Verfahren zur Herstellung von Dialkylaminophosphorfluoriden", German PAtent DE 664.438; filed July 3, 1935; issued Aug. 26, 1938 (also as US Patent 2,146,356, assigned to Winthrop Chemical Company, New York)
1936 While still with Henkel, Lange established contact to Procter and Gamble and assigned a patent to that company of Cincinnati, Ohio (US 2,258,500; Feb. 24, 1937; in Germany filed on March 2, 1936).
1937 Meanwhile, in the course of a systematic Buna-related (20a) work on esters and ester amides of phosphoric acid, which then were in commercial use for protection of plastics against aging etc., Schrader prepared several new such compounds (18) and his colleague Hans Kükenthal tested them for insecticidal activity to make sure that no useful property escaped their eyes. And some of them were highly toxic. The most effective compounds had two OH-groups of phosphoric acid substituted (ester type) by organic substituents, one more by an acidic group, while the double-bonded oxygen persists or may be replaced by sulfur. Effective acidic groups are Cl, F, SCN, CNO, CH3COO-, and others. This discovery led to the development of the highly toxic compound Tabun (20b), in which one of the hydroxy groups of the phosphate is replaced by a CN-group. Gerhard SCHRADER, assignor to Farbenfabriken Bayer, Leverkusen: "Verfahren zur Darstellung von N-substituierten Aminocyanphosphinsäure bzw. thiophosphinsäureestern.", German Patent DE 767,511; filed July 22,1937; published July 10, 1952
Schrader is often claimed to be the father of chemical warfare, yet his discovery was the result of a search for new and effective synthetic insecticides. As required by laws of the time, his discovery of the highly toxic substances had to be forwarded to the German Army weapons office (Heereswaffenamt) which decided about their further use - or abuse (21). In a letter of February 5, 1937, Schrader informed Professor Gross of Elberfelde about his discovery. The info was forwarded to the German Army Weapons Office. They asked Schrader to present his invention (21). However, Schrader´s employers regarded his discovery as deterrent for their main business, pharmaceutical drugs. As Hoffmann (19) explains, they didn´t want the name of their company to be associated with the development and manufacture of war agents. Therefore, Schrader´s laboratory was transferred to Elberfeld, into buildings considered since the early 1920´s only fit for demolition. Anyway, the Heereswaffenamt obtained the first samples of Tabun in May 1937, Sarin soon followed (20a). On June 18, 1937, Willy Lange´s authorization to teach at the University of Berlin was suddenly withdrawn by the Ministry of education, without any formal reason being given. (This was possible by a law which enabled the ministry of education to restrict or withdraw authorization to teach if the "interests of the university" would demand it). The university knew nothing about the decision and asked Lange to resume his duties, whereupon he informed his superiors of the dismissal.
1938 In the course of experiments to exchange the chlorine of dichlorophosphoric acid dimethylamide in the pesence of alcohol for fluorine (from sodium fluoride), Schrader realized to his surprise that not the corresponding fluoro compound did result but the diethyl ester of fluorophosphoric acid (Ref. 18, p. 14), though in much better yield than originally reported for another way of synthesis by Lange and Krueger. So he continued to synthesize a series of esters of fluorophosphoric acid, i. e. homologues (among them DFP) of the type of compounds which Lange and Krueger had described in 1932 and 1933: Gerhard SCHRADER, Hans KÜKENTHAL, assignors to Farbenfabriken Bayer, Leverkusen: "Bekämpfung tierischer Schädlinge", German Patent (DE) 767,153, filed August 2, 1938; granted September 20, 1951. Their patent, filed on August 2, 1938, but kept secret for a long time, was published only in September 1951. As opposed to some claims, this patent does NOT refer to Sarin or Sarin-type compounds! Lange continued his work in the field of fluorine chemistry at Henkel. A report on the synthesis of thiofluorophosphoric acids was published in "Chemische Berichte" still under his former address at the Berlin University (22), but the corresponding patent, wherein he proposed their use as insecticides, was assigned to Henkel & Cie: Willy LANGE, assignor to Henkel & Cie G.m.b.H., Duesseldorf: "Herstellung von Salzen der Thiodifluorphosphorsäure", German Patent DE 669,384; filed March 4, 1938, pat. Dec. 1, 1938 A note in his paper (22) says that due to certain circumstances his work had to be finished prematurely. In 1939, Lange and his wife emigrated from Germany via Canada to the United States where he continued to work for Procter & Gamble, Cincinnati.
1941 On Dec. 11th, 1941, Bernard Charles Saunders, University of Cambridge, UK, reported at a Ministry of Supply meeting in London the highly toxic nature of an ester of monofluorophosphoric acid, diisopropyl fluorophosphate (DFP), as a lethal inhalant. Inspired by the early report (1932) of Willy Lange, Saunders and his coworkers had prepared several new esters of monofluorophosphoric acid and had tested them for possible usefulness as warfare agents (23). The quick "knock-out" action of DFP was stated to be comparable with that produced by hydrogen cyanide. Another remarkable effect at much lower, and non-fatal, concentrations was stressed at the time: an intense constriction of the pupils of the eyes took place (miotic effect). Their initial observations led them to search for new and simple methods for the preparation of these compounds. Right at the start of the experiments reports were also made available to American workers (23,24).
1943 "Increasing interest in the development of new fluorine-containing substances" led, during World War II, to an association of Ozark Chemical Company (which possessed the raw material for fluorine compounds, fluorspar) with the Purdue Research Foundation (1) where research on some organic fluorine compounds was to be conducted for the National Defense Research Committee (25). "In the early 1940s, Mr. C. O. Anderson, who was the Ozark contact with Purdue, became acquainted with Dr. Willy Lange, who was at the University of Cincinnati after having emigrated from Germany. Lange had discovered and published on the fluorophosphoric acids while still in Germany (Lange 1927). In discussions with Lange the idea developed that there should be commercial applications for these new fluorine compounds, possibly as mothproofing agents, so he was retained as a consultant to help in a research and development program" (1) In the course of work supported by Ozark, the synthesis of monofluorophosphoric acid finally became possible. The preparation of fluorophosphoric acids became the subject of a dissertation by Ralph Livingston (May 1943). Livingston´s "Historical Review" attests to the fact that "a discussion of the fluorophosphoric acids resolves itself primarily into a discussion of a series of papers by W. Lange and coworkers which appeared over the years 1927 to 1935" (25a). In 1943, Willy Lange and Ralph Livingston filed a related patent assigned to Ozark Chemical Company: Willy LANGE, Ralph LIVINGSTON, Cincinnati, Ohio, assignors, by direct and mesne assignments, to Ozark Chemical Company, Tulsa, Oklahoma: "Anhydrous monofluorphosphoric acid and method of producing it"; US Patent 2,408,784, filed March 11, 1943, granted Oct. 8, 1946 (reacting anhydrous hydrofluoric acid and water-free metaphosphoric acid; "when poured in a cold concentrated aqueous solution of silver nitrate, heavy typical colorless crystals identifiable as silver monofluorphosphate are produced immediately. These crystals when reacted with methyl iodide form the characteristic ester as described in the literature. ... The product of our invention, to wit, anhydrous monofluorphosphoric acid is of value for the synthesis of various organic materials useful as insecticides, or as a catalyst and for other purposes in the arts, and it is therefore our belief that the acid which in consequence of our invention is now available will have numerous and important applications and that the invention marks a distinct and valuable contribution to industry"). In England, Saunders and McCombie of Cambridge, in cooperation with the Ministry of Supply filed patents on the preparation of esters of fluorophosphoric acid (e.g. DFP) via chlorophosphonic esters (a standard method for preparing organic fluoro compounds consists in treating a corresponding chloro-compound with inorganic fluoride, see also -> the FREONS): Hamilton McCOMBIE, Bernard Charles SAUNDERS, of Cambridge, and Charles Lawrence WHEELER, Ministry of Supply: "A process for the preparation of fluorophosphonic acids and chlorophosphonic esters", British Patent (GB) 601,210, filed Sept. 15, 1943, granted April 30, 1948 ("This invention relates to the preparation of esters of halogen phosphoric acids and it is one object of the invention to prepare highly toxic esters of fluoro phosphoric acid. It is believed, however, that the method is of general application to its preparation of esters of halogen phosphoric acids and that some of the less toxic compounds may be useful for such purposes as insecticides. Hitherto compounds of the type herein described have only been obtained by very complicated and devious methods." (emphasis added) ... The method consists of three steps: (a) selection of phosphorus trichloride for reaction with the hydroxy body or mercaptan by which the respective alkyl hydrogen phosphite is obtained; (b) treatment of the product obtained in step (a) with chlorine to produce the chloro-phosphoric ester; (c) preparation of highly toxic fluorine compounds by heating gently the chloro compound (step (b)) with excess metallic fluoride (e.g. sodium fluoride) and distilling the organic fluorine compound from the chlorine residue. Hamilton McCOMBIE, Bernard Charles SAUNDERS, Norman Bellamy CHAPMAN, Robert HEAP, University of Cambridge, and James Davidson PRATT, Ministry of Supply: "A process for the production of fluorophosphonic acid compounds", British Patent (GB) 602,446; filed April 17, 1944, granted May 27, 1948
1944 Hardy and Kosolapoff, assignors to Monsanto Chemical Company, patented the production of dialkyl fluorophosphates (such as DFP, diisopropyl fluorophosphate) "by a process which comprises reacting an aliphatic alcohol with phosphorus trichloride to form a dialkyl hydrogen phosphite, chlorinating the crude product and then fluorinating the dialkyl chlorophosphate formed by means of an alkali fluoride (e.g. sodium fluoride) to produce the corresponding dialkyl fluorophosphate": Edgar E. HARDY, Gennady M. KOSOLAPOFF, assignors to Monsanto Chemical Company: "Halogenated compounds and process for making same", US Patent 2,409,039; filed Jan. 28, 1944; granted Oct. 8, 1946 Willy Lange, Cincinnati, Ohio, filed another patent on the production of anhydrous monofluorophosphoric acid: Willy LANGE, Cincinnati, Ohio, assignor, by direct and mesne assignements, to Ozark Chemical Company, Tulsa, Oklahoma: "Method of production of anhydrous monofluorophosphoric acid"; US Patent 2,408,785; filed June 28, 1944; granted Oct. 8, 1946 ("Monoethyl-monofluorophosphoric acid, obtained e.g. by reacting the anhydrous acid with ethylene, forms diethyl monofluorophosphate in a reaction corresponding to that of monoethyl sulfuric acid which, when heated, forms diethyl sulfate" Possible uses of the (mono- and di-) fluorophosphoric acids include "as catalysts for polymerization, condensation and alkylation reactions especially for combining such compounds as isoalkanes, and olefines or isoolefines. Esters may be prepared by reacting olefinic or acetylenic compounds with the anhydrous acids in the presence of catalysts, or by reacting alcohols or ethers with the anhydrous acids...")
1946 Ozark Chemical Company and Mahoning Mining merged into the "Ozark Mahoning Company (1). Willy LANGE, Cincinnati, Ohio, and Ralph LIVINGSTON, Oak Ridge, Tennessee, assignors to Ozark-Mahoning Company: "Method of producing hexafluorophosphoric acid"; US Patent 2,488,298; filed Sept. 12, 1946; granted Nov. 15, 1949
1947 Willy Lange (Procter & Gamble Company) and Ralph Livingston (now with Monsanto Chemical Company, Clinton Laboratories, Oak Ridge, Tennessee) published the 13th report in a series of studies of fluorophosphoric acids: Preparation of anhydrous monofluorophosphoric acid (25b). "It has recently been shown that the esters of monofluorophosphoric acid which were previously known to be highly toxic, have a probable usefulness in the treatment of glaucoma and myasthenia gravis, the di-isopropyl ester being especially suited for this purpose. McCombie and Saunders have devised two convenient methods for the synthesis of these esters, but their prospective uses make a cheap direct synthesis from monofluorophosphoric acid seem desirable." Carl O. Anderson, of the Ozark Mahoning Company, filed a patent for the production of potassium and sodium monofluorophosphates from potassium/sodium fluorides (to avoid the use of HF) and potassium/sodium metaphosphates. This Ozark patent, granted in 1949, still did not advise use of MFP in toothpaste: Carl O. ANDERSON, assignor to Ozark Mahoning Company, Tulsa, Oklahoma: "Method of producing monofluorophosphates"; US Patent 2,481,807, filed September 28, 1947, patented Sept. 13, 1949 ("Commercial uses of these monofluorophosphates are as yet not highly developed, due probably in large part to the lack heretofore of a practical commercial method for their production in quantity, but researches thus far completed indicate they may have wide application in medicine, insecticides, mothproofing, electrochemical processes and other fields"). Willy LANGE, Cincinnati, Ohio, and Ralph LIVINGSTON, Oak Ridge, Tennessee, assignors to Ozark Mahoning Company, Tulsa, Oklahoma: "Method of producing hexafluorophosphoric acid"; US Patent 2,488,299; filed Dec. 4, 1947; granted Nov. 15, 1949
1948 Saunders and Stacey, of Cambridge, UK, described the preparation of diisopropyl fluorophosphonate (DFP), "in excellent yield", by conversion of the chlorophosphonate.by means of sodium fluoride (24). Their two British patents had just been granted when they filed a US Patent on their topic: Hamilton McCOMBIE, Bernard Charles SAUNDERS, Norman Bellamy CHAPMAN, Robert HEAP: "Process for the production of fluorophosphonic acid compounds", US Patent 2,489,917; filed Feb. 25, 1948, granted Nov. 29, 1949 "Industrial and Engineering Chemistry" announced a "New Baby in the Fluorine Family", another "milestone in the history of fluorine chemistry": the availability of salts of monofluorophosphoric and hexafluorophosphoric acids (generally designated FP salts by Ozark). Their history, the report says, "is known to those familiar with the researches of Willy Lange and his co-workers in the years 1928 to 1933. Commercial production by Dr. Lange´s methods as published in German journals was too costly and tedious to be practicable, but more recently Dr. Lange, who came to the United States in 1939, has secured patents on methods of more commercial significance; these patents are assigned to the Ozark-Mahoning Company, Tulsa, Okla. The acids were announced briefly by Ozark in 1944, but shortage of personnel and equipment greatly retarded further developments at that time" (24a). It was well realized that methods of preparation using anhydrous hydrogen fluoride spell "trouble", but "Ozark´s chemists have licked these problems". Stability, storage and shipment were no longer big problems. Concerning toxicity, the report claims: "Toxicity of the FP compounds is not marked, although it may be more than that of simple fluorides. They do not possess the intense physiological action of the dialkyl esters of the mono- acid, such as DFP (diisopropyl fluorophosphate), which has become of importance in recent years in the treatment of glaucoma and has been described, apparently incorrectly (though conceivably) as composing the German "nerve" war gases known as Tabun and Sarin." - An early attempt of white-wash, apparently, for DFP is not Tabun nor Sarin, but one of the nerve agents nevertheless.
1949 Research Council Meeting at the Army Chemical Center, Maryland, Feb. 17, 1949 (from DOE Openness, Human Radiation Experiments website). Among the members of the Research Council: Harold C. Hodge, fluoride expert at the University of Rochester. The Atomic Energy Commission, according to the meeting minutes, "more recently released considerable information on fluorine compounds", F2O was considered even more toxic than the "G" compounds (nerve agents), but too difficult to package because of the extremely low critical temperature. But "Monsanto Company has a contract to design a plant to make GB, which should be ready in 1950". (GB was the codename given to Sarin). One of the possible reasons why Hitler did not deploy nerve agents during World War II, was that there was no effective protection available for his army soldiers who could become exposed to the agents (19b, pp. 422-423). Thus, among the American researchers who met at the Army Chemical Center, a third project in the 1-C priority group was "on adsorption ... we are doing nothing in the laboratory, but the University of Rochester is continuing contract work, particularly in respect to charcoal on clothing and in respect to kinetics of charcoal on clothing." Willy Lange, Cincinnati, Ohio, and Archie Hood, Tulsa, Oklahoma, file a patent on a simple and effective way to produce mono- and di-alkyl monofluorophosphates (now quite elegantly circumscribed as "mono and di- hydrocarbon-oxy phosphoryl monofluorides"): Willy LANGE, Cincinnati, Ohio, and Archie HOOD, Tulsa, Oklahoma, assignors to Ozark Mahoning Company, Tulsa, Oklahoma: "Production of hydrocarbon-oxy phosphoryl monofluorides", US Patent 2,614,116; filed Feb. 23, 1949; granted Oct. 14, 1952. ("The literature contains reports of laboratory preparation by somewhat complicated procedures of certain dialkoxy phosphoryl monofluorides; the diisopropyl compound (PM: diisopropyl monofluorophosphate, DFP) has been produced on a fairly large scale and certain physiological experiments have been made with it to determine its effects on the nervous system while other research has been directed to its exploitation in treatment of human diseases and other abnormal conditions." The authors prepare the alkyl monofluorophosphates by treating alkyl polyphosphates with hydrogen fluoride. In contrast to the dialkyl derivatives, the mono-esters are claimed to have a low toxicity for mammals but "show strong fungicidal properties" and may be "suitable compounds for use in inhibiting growth of mold in or on materials treated with them.") Heinz JONAS, assignor to Farbenfabriken Bayer, Leverkusen: "Herstellung von Hexafluorphosphorsäure und deren Salzen", German Patent DE 812,247; filed March 31, 1949; patented June 28, 1951
1950 Willy Lange and Ralph Livingston reported on the preparation of anhydrous difluorophosphoric acid. Their objective was achieved by allowing phosphorus oxytrifluoride to react with anhydrous monofluorophosphoric acid. This work, a portion of a thesis submitted by Livingston in 1943, "was done under a graduate cooperative fellowship (R.L.) sponsored by the Ozark Chemical Company, now Ozark Mahoning Company, Tulsa Oklahoma" (26). In their search for possible commercial applications, Ozark Mahoning contacted Harold C. Hodge at the Rochester University with the request that he check the toxicity of the new complex fluoride Ozark had learned to make, sodium monofluorophosphate (1), for "First, there was the idea that had originated with Dr. Lange that a fluorophosphate should be effective as a mothproofing material and might therefore be toxic also to humans. But a more compelling basis for the belief that this might be quite poisonous was the knowledge that when the PO3F2- group is combined with two isopropyl groups to form the ester, diisopropyl-monofluorophosphate, known during and after World War II as DFP, it is a potent inhibitor of choline esterase and hence is one of the ´nerve gases´" (1). According to White, Hodge arranged to have that test done and "fortunately his relationship with the Dental School and with two graduate students in that school was such that the study included a test on anticaries activty." White met Hodge, Hein and others several months later at the IADR conference in French Lick, Ind., when John William Hein presented the results of the tests sponsored by Ozark Mahoning Company and Eastman Dental Dispensary (28). Lange heard in a private communication from Ozark of the relatively low toxicity of sodium monofluorophosphate and its possible usefulness in dentistry, but he found the latter "has not been explored sufficiently to permit conclusions" (27). A chapter on the chemistry of the fluoro acids published in "Fluorine Chemistry. Volume I" (27), is apparently Lange´s last (public) contribution to the field of fluorine chemistry. Several patents filed by him between 1951 and 1965 and assigned to Procter and Gamble cover fields unrelated to fluorine.
1951 Loren C. Mosier and Wayne E. White of Ozark Mahoning Company presented a plan of a pilot plant for production of "experimental quantities" of the fluorophosphoric acids and described parts of the processes (29).
1952 Albertus J. MULDER, Willem C. Brezesinska SMITHUYSEN, assignors to Shell Development Company, Emeryville, Ca.: "Hexafluorophosphoric acid compositions and preparation thereof", US Patent 2,718,456; filed March 17, 1952; patented Sept. 20, 1955
1953 Archie HOOD, Houston, Tx., assignor to Ozark-Mahoning Company, Tulsa, Oklahoma: "Method of producing monohydroxy monohydrocarbonoxy phosphoryl monofluorides", US Patent 2,712,548; filed Sept. 3, 1953; granted July 5, 1955 (= production of mono-esters of monofluorophosphoric acid)
1954 During 1954 to 1957, certain data was published -by the stannous fluoride research group at the University of Indiana- indicating the relative ineffectiveness of sodium monofluorophosphate in the inhibition of dental caries. It was reported in the Journal of Dental Research, Oct. 1954, p. 676 (J.C. Muhler, H. G. Day), that dental caries studies on animals disclosed that sodium hexafluorophosphate was effective and sodium monofluorophosphate was ineffective (in sharp contrast to what the Rochester group reported in 1951 (29a)). In 1956 and 1957 it was reported that sodium monofluorophosphate was essentially ineffective on certain tooth structure (30). These researchers at the University of Indiana, who developed several tin fluoride compounds for use in toothpaste, enjoyed the support of a loyal former colleague, Arthur W. Radike, a University of Indiana alumnus who was now heading the Drug Development Section of Procter and Gamble Company, which decided to sponsor the tin fluoride toothpaste research (30a) which led to "Crest".
1955 In their patents on "Dentifrice Preparations", Virgil John Richter and Roderick David Manahan of Colgate-Palmolive Company mentioned the possibility of using aluminum mono- and di-fluophosphates, or the sodium or potassium fluophosphates as a fluoride source in toothpaste, but their examples for preparations as well as their claims only referred to sodium or stannous fluoride: Colgate-Palmolive Company: "Préparation dentifrice", French Patent (FR) 1,124,756; filed April 25, 1955, granted Oct. 17, 1956 Roderick David MANAHAN, Virgil John RICHTER, assignors to Colgate-Palmolive Company: "Dentifrice Preparations", Australian Patent (AU) 217,073; filed April 20, 1956, granted Oct. 25, 1956 Roderick David MANAHAN, Virgil John RICHTER, assignors to Colgate-Palmolive Company: "Fluoride Dentifrice Composition", US Patent 3,227,617; filed March 14, 1957; granted Jan. 4, 1966
1956 On June 20, Colgate-Palmolive Company formally established a Division of dental medicine at Rutgers University. John William ("Jack") Hein was appointed director of the new division at the school in New Brunswick, N.J. (31). Hein continued his support for MFP research at Colgate (1).
1957 The Rochester group, with coauthor John W. Hein, reported that sodium monofluorophosphate, like sodium fluoride, reduces periodontal disease in male hamsters given 40 ppm fluoride in the drinking water. Coauthor John W. Hein then had become dental director, Colgate-Palmolive (32).
1959 While Procter and Gamble as well as Colgate-Palmolive were still reluctant regarding the use of sodium monofluorophosphate in toothpaste, Sten Yngve Ericsson of Sweden, a representative of dentistry in the World Health Organization, filed a patent on a toothpaste containing monofluorophosphate: Sten Yngve ERICSSON, Föreningen Svenska tillverkare av Fluortandkräm, Stockholm (transl.: "The Association of Swedish Manufacturers of Fluoride Toothpaste, Stockholm"): "Tandbehandlingsmassa innchallande fluorförening och slipmedel", Swedish Patent (SE) 222,895; filed August 7, 1959; granted Oct. 1, 1968 Sten Yngve ERICSSON, Djursholm: "Alkali Metal Monofluorophosphate and Calcium Carbonate Dentifrice", US Patent 3,119,743; filed Aug. 4, 1960; granted Jan. 28, 1964 (claims that neither calcium carbonate (abrasive) nor the carboxymethyl cellulose (increasing the viscosity) or the sodium lauryl sulphate (detergent) inactivate the fluorine of the monofluorophosphate). His patents apparently were realized with "Pepsodent" (33).
1960 Wayne E. WHITE, assignor to Ozark Mahoning Company, Tulsa, Oklahoma: "Fish Control Process"; US Patent 3,076,743; filed August 30, 1960; patented Feb. 5, 1963; (benzyltrimethylammonium hexafluorophosphate and the hexafluoroarsenate are proposed for the control of carp and other bottom feeding fish species)
1961 Richard JOHNSON, Frederick L. HORN, Gerald STRICKLAND, assignor to the United States of America as represented by the United States Atomic Energy Commission: "Dissolution of Uranium fuels by mono- or difluorophosphoric acid", US Patent 3,088,800; filed Aug. 30, 1961; patented May 7, 1963 Kenneth Rutherford et al. suggest hexafluorophosphoric acid as a useful reagent for the conversion of aromatic amines to fluorinated aromates in a modified Schiemann Reaction with marked improvement over the use of fluoroboric acid (34).
1962 "Fluorine babies grow up" reports the August 1962 issue of the Journal "Industrial and Engineering Chemistry" (35). Sodium monofluorophosphate is being used "regularly or experimentally in dental preparations manufactured in Hong Kong, Formosa, Switzerland and Sweden, as well as the U.S. ... Difluorophosphoric acid has been sold now for several years on a small scale as a polymerization catalyst. ..." Concerning hexafluorophosphoric acid the report refers to Compound 191, a carp control agent consisting of a substituted ammonium hexafluorophosphate (as patented by Wayne E. White in 1960), for which "indications point to a rather sizeable consumption". In cooperation with researchers at the Oklahoma School of Medicine, Wayne E. White examined the "effect of daily applications of sodium monofluorophosphate solution on caries rate in children" and claimed a "significant additive, anticariogenic effect of sodium monofluorophosphate to that accruing from water fluoridation". This investigation was supported in part by a grant from the National Institute of Dental Research and in part by Ozark-Mahoning Company (36). The study design: one group of students (6 to 14 years) of a small parochial school in a city with fluoridated water was given a solution containing 6 per cent sodium monofluorophosphate, a second group was given the same solution but with sodium chloride substituted for the mfp. All participants were asked to use the solutions once daily for brushing. It was requested that none of the subjects use a dentifrice containing a fluoride or have a topical fluoride treatment during the study period. Clinical and radiographical examinations were performed at the start and at certain intervals (on decreasing numbers of subjects participating). Data were scored in this unsupervised study as relative increment of decay (R.I.D.), which is the percentage change of the ratio of number of surfaces becoming carious or filled to the number of surfaces at risk.
1963 In May and June, 1963, Wayne E. White, of Ozark-Mahoning, "traveled in England, Denmark, Norway, Sweden, Germany, Italy, France, and Holland" to find a market for MFP, "and upon my return to Tulsa I reported to our directors that within a year there might be an annual consumption of as much as 90,000 lb. by the dentifrice manufacturers in the countries I had visited" (White W. E. 1983, Ref. 1). Starting in 1963, Manahan and Richter, of Colgate-Palmolive, filed a series of patents on the use of sodium monofluorophosphate in toothpaste: Roderick David MANAHAN, Virgil John RICHTER: "Zahnpflegemittel", Swiss Patent (CH) 438,586; filed March 1, 1963; granted June 30, 1967 Roderick David MANAHAN, Virgil John RICHTER: "Dentifrice Preparation", British Patent (GB) 1,004,039; filed March 4, 1963; granted Sept. 8, 1965 Roderick David MANAHAN, Virgil John RICHTER: "Zahnpflegemittel", Swiss Patent (CH) 454,359; filed July 13, 1964; granted April 15, 1968 Roderick David MANAHAN, Virgil John RICHTER: "Dentifrice Composition containing sodium monofluorophosphate", US Patent 3,227,618; filed March 9, 1964; granted Jan. 4, 1966 Roderick David MANAHAN, Virgil John RICHTER: "Dentifrice preparation", US Patent 3,634,585; filed June 24, 1964; granted Aug. 11, 1966 Even Monsanto concurred with a related patent: Elerington SAUNDERS, Thomas SCHIFF, assignors to Monsanto Company: "Dentifrice compositions comprising dicalcium orthophosphate and sodium monofluorophosphate", US Patent 3,308,029; filed Nov. 6, 1963; granted March 7, 1967
1965 To eventually keep step with an increase in the demand of MFP, more patents on its preparation were issued and Rolf Wittmann, of Merck Company, did not even hesitate to consider the use of monofluorophosphate (mono)esters as toothpaste additives: Rolf WITTMANN, assignor to E. Merck A.G. Darmstadt, Germany: "Verfahren zur Herstellung von Monofluorphosphorsäure und deren Monoestern", German Patent (DE) 1,264,423; filed Jan. 27, 1965; granted March 28, 1968; and Swiss Patent (CH) 498,047; filed Oct. 27, 1965; granted Oct. 31, 1970; French Patent (FR) 1,464,472; filed Jan. 19, 1966; Rolf WITTMANN, assignor to E. Merck A.G. Darmstadt, Germany: "Process for the manufacture of monofluorophosphoric acid and its monoesters", US Patent 3,424,550; filed Jan. 20, 1966; granted Jan. 28, 1969 ("The monofluorophosphoric acid and its monoesters can be advantageously used in dentistry, for example, as additive in toothpastes or similar preparations for prevention of caries.")
1966 Ozark-Mahoning´s Wayne E. White et al. patented a method and apparatus for the continuous production of sodium monofluorophosphate: Wayne E. WHITE, James M. MUNN, Joe E. GILLILAND, Benny B. WRIGHT, assignors to Ozark-Mahoning Company: "Process and apparatus for production of alkali metal monofluorophosphate"; US Patent 3,463,605; filed June 13, 1966; granted Aug. 26, 1969 ("Sodium monofluorophosphate has been found useful in dental preparations, in dentifrices as a caries preventative and in solutions as a caries preventative and tooth desensitizer. Sodium monofluorophosphate has also been used as a mold inhibitor.")
1968 John D. NICKERSON, Robert A. WIESBOECK, assignors to United States Steel Corporatioon, Pittsburgh, Pa: "Process for preparing phosphorus pentafluoride and fluorophosphoric acids", US Patent 3,634,034; filed Feb. 2, 1968; patented June 11, 1972
1969 Sten Yngve Ericsson filed a British Patent for the use of monofluorophosphate to harden teeth and bones: Sten Yngve ERICSSON: "Compositions for hardening teeth and bones", British Patent (GB) 1,221,633; filed Jan 13, 1969; granted Feb. 3, 1971
1975 Albright & Wilson Limited: "Method for the manufacture of alkali metal monofluorophosphate", British Patent GB 1,544,197; filed Jan. 14, 1975; patented April 11, 1979
1985 MFP was proposed as a corrosion inhibitor for protecting metallic surfaces which are in contact with water: Francis MORAN, Sylvain ROCHER, Louis COT, FRancis DABOSI, Michel DUPRAT, Jean DURAND, assignors to Union Chimique et Industrielle de l´Ouest, France: "Anticorrosion means and compositions containing same", US Patent 4,613,450; filed March 25, 1985; granted Sept. 23, 1986
1987 Frank MOEWIUS, Manfred MEISEL, Herbert GRUNZE, Lothar KOLDITZ, Marina ZELBIG, Walfired OESE, Dietmar STANDFUSS, Horst KIRK, Reiner HESSE, Horst GOETZE, Wibke UNGER, assignors to Desowag Materialschutz, GmbH, Düsseldorf, Germany: "Wood preservative composition and use thereof", US Patent 4,767,458; filed Sept. 15, 1987; patented Aug. 30, 1998
1989 Richard TÄNZLER, Alexander MAURER, Armin ETZEL, Hans-Georg STEINERT, assignors to Benckiser-Knapsack GmbH: "Vorrichtung und Verfahren zur Herstellung von Alkalimetallmonofluorphosphat", European Patent EP 362,746; filed Sept. 30, 1989; patented April 11, 1990
1990 Marcus G. GRODBERG, assignor to Colgate-Palmolive Company: "Composition and method for preventing fluorosis", US Patent 5,153,005; filed Dec. 11, 1990; granted Oct. 6, 1992; and European Patent EP 494,512; filed Dec. 9, 1991; patented July 15, 1992 Hans Walter SWIDERSKY, Werner RUDOLPH, Ulrich HARTMANN, Frank MOEWIUS, Veronika RADONZ, Manfred MEISEL, assignors to Kali Chemie Aktiengesellschaft, Hanover, Germany: "Method for the manufacturing of alkali monofluorophosphate", US Patent 5,393,506; filed Dec. 24, 1990; patented Feb. 28, 1995
1991 MFP described as a solubility inhibitor for lead in potable water sources: Bennet P. BOFFARDI, Ann M. Sherbondy, assignors to Calgon Corporation: "Monofluorophosphate solubility inhibitor for lead in potable water sources", European Patent (EP) 481,669; filed Oct. 9, 1991; granted April 22, 1992 ("when added to potable water sources in concentrations between 0.1 mg/l and 500 mg/l, significantly reduces lead (Pb) leaching into said water, producing the double benefit of reducing lead solubility and thus content in drinking water, while at the same time adding fluoride to that drinking water, with the anticaries benefit that fluoridation provides.")
1992 Bennett P. BOFFARDI, Ann M. SHERBONDY, assignors to Calgon Corporation: "Monofluorophosphate for calcium carbonate scale control and iron and manganese stabilization", European Patent EP 506,339; filed March 24, 1992; patented Sept. 30, 1992
1999 Due to "increased costs associated with compliance with new FDA regulations for bulk pharmaceutical intermediates", Ozark-Mahoning adjusted the price of its dentifrice fluoride product, USP-grade sodium monofluorophosphate (SMFP) to $3.13 per kilogram (http://www.findarticles.com/m0FVP/9_256/55621993/p1/article.jhtml)
References: (1)Wayne E. White, Ozark-Mahoning Company, in Caries Res. 17: Suppl. I (1983) pp. 2-8, "Monofluorophosphate - Its Beginning"; (2) J. Dent. Assn. South Africa (Dec. 1980) p.837; (3) Brit. Dent. J. 150 (1981) 285; (4) Willy Lange collection, Archives and Rare Books Library, University of Cincinnati; (4a) Environ. Sci. Technol. 4 (1970) 725; (5) Nature 215 (1967) 1277; (6) J. Agric. Food Chem. 18 (1970) 789; (7) C. R. 103 (1886) 1257; H. Moissan: "Das Fluor und seine Verbindungen", 1900, p. 172; (8) Weinland H.F., Alfa J.: Ber. dt. chem. Ges. 31 (1898) 123; (9) Z. anorg. Chem. 21 (1899) 43; (9a) Seifert 1932, cited in "Fluorine Chemistry", Vol. 1, (J. H. Simons (ed.)), Academic Press, New York 1950, p. 147; (10) Lange W.: "I. Über die bei Einwirkung von konzentrierter Schwefelsäure auf Flusspat sich abspielenden Reaktionen. II. Beiträge zur Kenntnis der Fluorsulfonsäure", Inaug. Diss., Berlin 1923; (11) German Patent DE 342,898; (12) German Patent DE 346,245; (13) Ber. dt. chem. Ges. 60 (1927) 962); (14) Ber. dt. chem. Ges. 61 (1928) 799; (15) Ber. dt. chem. Ges. 62 (1929) 793 and 1084; 64 (1931) 2772; (15a) W. Lange to John P. DeCamp, Sept. 15, 1965, in the Willy Lange Papers, Archives and Rare Books Library, University of Cincinnati; (16) Ber. dt. chem. Ges. 65 (1932) 1598; (-> History of the nerve agents); (17) Gerda v. Krueger, "Zur Kenntnis einiger Phosphor und Fluor enthaltender Verbindungen", Inaug. Diss., Berlin 1933; (17a) Laties A.M.: Investigative Opthalmology 11:7 (July 1972) 555; (18) Schrader G.: "Die Entwicklung neuer Insektizide auf Grundlage organischer Fluor- und Phosphor-Verbindungen", Verlag Chemie, Weinheim, 1952, pp.5-9; (19) Hoffmann H.: "Erinnerungen", unpublished Manuscript; (19a) Angew. Chem. 38 (1925) 441; (19b) Deichmann U.: "Flüchten, Mitmachen, Vergessen. Chemiker und Biochemiker in der NS-Zeit", Wiley-VCH, Weinheim 2001, pp.120, 124; (20) Henkel & Cie GmbH: "Gedenkschrift für Hugo Henkel", Düsseldorf 1952; (20a) Grümmer G.: "Giftküchen des Teufels", Militärverlag der Deutschen Demokratischen Republik, Berlin 1985, pp. 114 ff.; (20b) Ref. 18, pp. 9-26; (21) Pfingsten O.: "Dr. Gerhard Schrader", Wendeburger Heimatkunde, Heft 24, Verlag Uwe Krebs, Wendeburg 2003; (22) Chem. Ber. 71 (1938) 801; (23) Saunders B. C.: "Some aspects of the chemistry and toxic action of organic compounds containing phosphorus and fluorine", Cambridge, University Press 1957, p. 42; (24) J. chem. Soc. (1948) 695; (24a) "New baby in the fluorine family", Ind. Eng. Chem. 40 (Sept. 1948) pp. 22a & 24a; (25) National Defense Research Committee: "Final Report on ´Fluorocarbons and related compounds´", by Albert L. Henne, Report OSRD No. 1792, Sept. 10, 1943, unclassified Sept. 18, 2001; (25a) Ralph Livingston: "Fluorophosphoric Acids", Dissertation submitted to the Faculty of the Graduate Department of Applied Science, College of Engineering and Commerce, University of Cincinnati, in partial fulfillment of the requirements for the degree of Doctor of Science, May 1943; (25b) J. am. chem. Soc. 69 (1947) 1073; portion of a thesis by R. Livingston, 1943, sponsored by Ozark Chemical Company; (26) J. am. chem. Soc. 72 (1950) 1280; (27) "Fluorine Chemistry, Vol. I", J.H. Simons (ed.), Academic Press, New York 1950, p.125; (28) Harold Carpenter Hodge, Kanwar Shourie, and NIH senior research fellow at Rochester John W. Hein, J. dent. Res. 29 (1950) 529; (29) Mosier L.C., White W.E.: "Fluorine Chemicals", Ind. Eng. Chem. 43 (Jan. 1951) 246; (29a) Hein et al.: J. dent. Res. 30 (Aug. 1951) 467; (30) J. dent. Res. (Feb. 1956) 59-64, and (1957) 889-894; cf. Manahan and Richter, US Patent 3,227,618 filed 1964, granted 1966; (30a) Necrology: Joseph C. Muhler, http://www.chem.indiana.edu/Alumni/pubs/w97/necrology.html (May 20, 2002); (31) "Colgate Research Division established at Rutgers", J. Am. Dent. Assn. 53 (Aug. 1956) 257; (32) J. Am. Dent. Assn. 55 (Nov. 1957) 617; (33) Bruker M.O., Ziegelbecker R.: "Vorsicht Fluor", emu-Verlag, 1995, p. 81; (34) Rutherford K. et al.: J. Org. Chem. 26:12 (1961) 5149; (35) "Fluorine babies grow up", Ind. Eng. Chem. 54:8 (1962) 18; (36) Goaz PW, McElwaine LP, Biswell HA, White WE: J. dent. Res. 42 (1963) 965; ibid. 45 (1966) 286;
* The helpful support by the very kind people of the University of Cincinnati´s Archives and Rare Books Library is gratefully acknowledged.
|
|||||||